Diamond D Ultra Impact Denture Acrylic by Keystone Industries is Proud to be Made in the USA in Keystone Industries Cherry Hill New Jersey Plant. Documentation Available
Diamond D Ultra Impact Denture Acrylic is the premium denture polymer proven to meet ADA/ANSI Specification #12 requirements for Denture Base Polymers. Diamond D High Impact Denture Acrylic received their 510(k) NO: K032773 on 09/23/03. It is manufactured by Keystone Industries in their cGMP, ISO 9001:2000 and ISO 13485:2003 compliant facility located in Cherry Hill, New Jersey USA. Not all Hi-Impact Denture Acrylics are manufactured in ISO compliant facilities, is yours? Ask for documentation.

We are doing business in a continuously growing litigious society. Dental Laboratories that manufacture these regulated medical/dental devices should consider purchasing the products used in the manufacturing of these devices from manufacturers that have ISO 9001:2000 registered manufacturing facilities. Easily accessible documentation and traceability required and all ready in place from these manufacturers should make this obvious. At the ADA website under "American Dental Association Statement on Safety Concerns with Dental Prosthesis" already suggests that Dentists ask the laboratories they use these questions.
>Do you produce your own crowns, bridges and other dental materials in the office or purchase them from a dental laboratory?
>Where is the dental lab located?
>Does the lab outsource crowns or bridges to a foreign country?
>If the lab is in a foreign country, does it provide written documentation that it is registered with the FDA?
>Does the lab provide written documentation that it uses FDA-approved materials?
>Have you noticed any problems with the crowns, bridges or other items produced by this dental lab?
>What other options do I have?
Dental laboratories will need to respond immediately to these requests for information and documentation.
Diamond D High Impact Denture Acrylic received their 510(k) NO: K032773 on 09/23/03.
Diamond D Denture Acrylic contains no cadmium, phthalates or bisphenol A (BPA)
Since Diamond D Ultra Impact Denture Acrylic is the premium denture polymer proven to meet ADA/ANSI Specification #12 requirements for Denture Base Polymers and is manufactured by Keystone Industries in their cGMP, ISO 9001:2000 and ISO 13485:2003 compliant facility located in Cherry Hill, New Jersey USA. The Denture Acrylic of choice is obvious.
To learn more why Diamond D Ultra Impact Denture Acrylic is the most fracture resistant heat cure denture acrylic on the market and should be your denture acrylic of choice go the the Diamond D landing page at www.keystoneind.com/html/web-content/diamond_d.html and click on testing data.
###
Carl Rogers
800 333-3131
www.keystoneind.com
Dental Acrylics
616 Hollywood Ave
Cherry Hill, NJ 08002
Fax No 856 663-0381
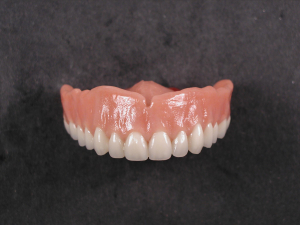
Diamond D Ultra Impact Upper Denture
An upper denture plate made by Matrix Dental Laboratory in Crown Point Indiana
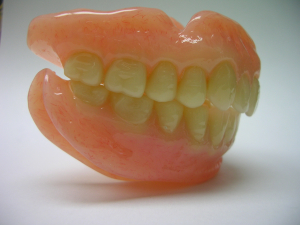
Upper and Lower Diamond D Denture
An upper and lower Denture processed with Diamond d Ultra Impact Denture Acrylic by Alex Hupka
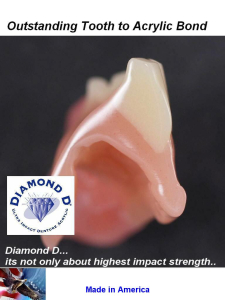
Diamond D Tooth Adhesion
Cross Cut picture of the Diamond D Ultra Impact Denture Acrylics phenomenal adhesion to acrylic teeth. Bonds as if one piece and no separation can be felt or seen.
