The Next Generation of Vivace® is Announced, Introducing Vivace Ultra™
The Beginning of a New Era with Unmatched Precision, Vision, and Technology.
Phoenix, AZ, July 08, 2022 --(PR.com)-- Aesthetics Biomedical® is creating an entirely new category that redefines what is possible in personalized medical aesthetics, offering a novel approach to clinical precision and treatment delivery, elevating consumer outcomes and experience.
Vivace Ultra™, now awaiting FDA Clearance, provides physicians and aesthetic practitioners unmatched precision, visualization, and the most versatile radiofrequency (RF) microneedling technology available. An innovative reimagination and technical upgrade of the legacy Vivace® Microneedle RF treatment and device, the Vivace Ultra™ combines two unique modalities into one compact device, delivering the most bespoke, personalized treatment experience possible. This new era of advanced energy-based technology offers data-supported treatment parameters made possible by an industry-first ultrasound imaging and mapping, an exclusive uniform radiofrequency microneedling energy delivery system, range of frequency options, and a HIPAA compliant patient data-tracking system for optimal consumer results and treatment plans. Without a doubt, Vivace Ultra™ is the next generation of the Vivace® Microneedle RF.
A recent clinical study found that 95% of patients noticed an improvement in skin texture after a series of treatments between 2-6 sessions. 94% of these patients said they would recommend radiofrequency microneedling to a friend1. On the RealSelf platform, the legacy Vivace® Microneedle RF treatment has become the most requested microneedling treatment2. Building upon an already best-in-class device, Vivace Ultra™ has been developed alongside and in collaboration with key opinion leaders. The new device’s technology and user-friendliness is based on years of clinical and user feedback from top plastic surgeons, dermatologists and medical aesthetic practitioners.
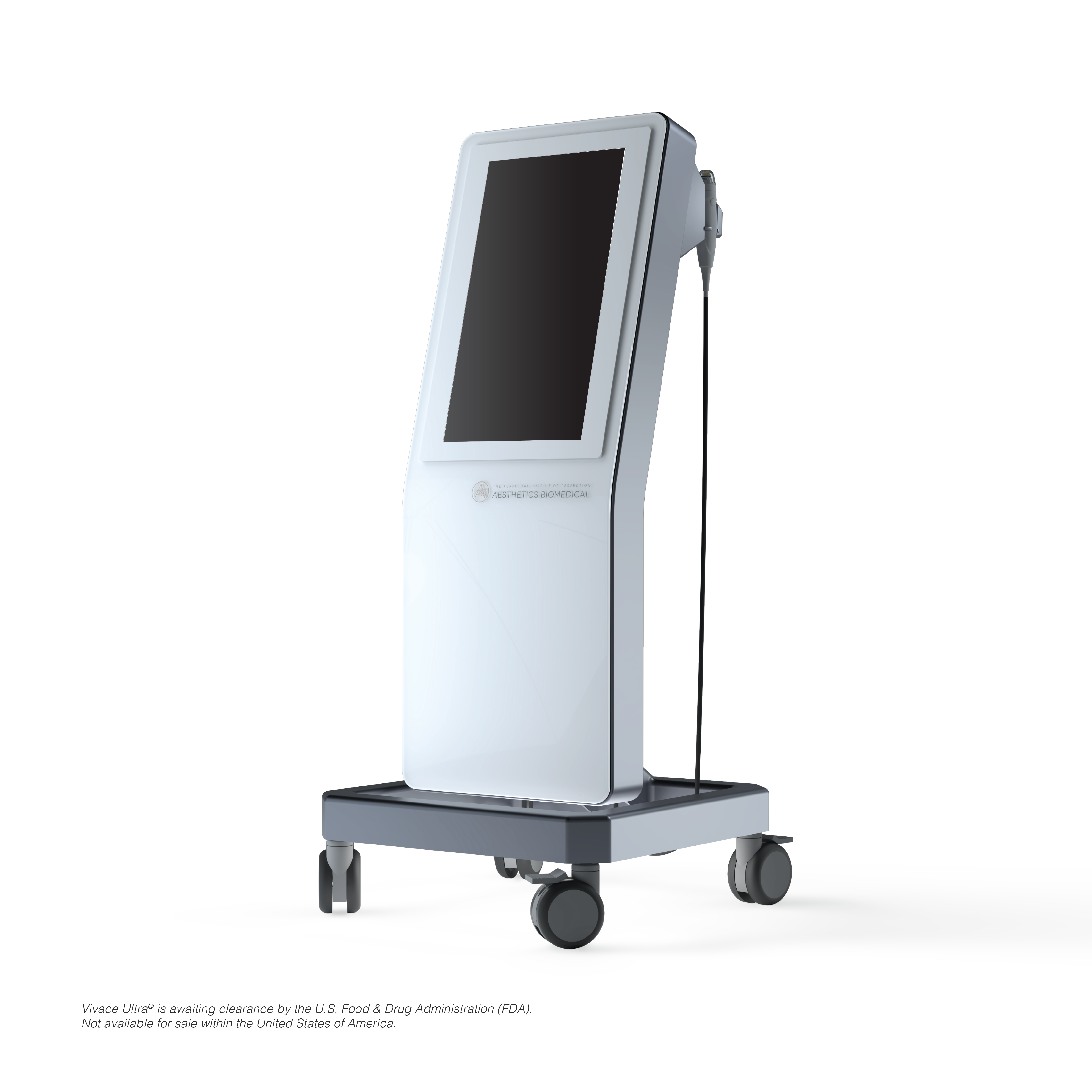
Sleek and simplistically styled, the Vivace Ultra™ is developed and manufactured by Aesthetics Biomedical® Inc., leading the market as the first and only RF Microneedling device manufactured in the United States of America, Phoenix, Arizona. Vivace Ultra™ uses linear array ultrasound technology to visually map the skin across its large 21.5" display screen, allowing an aesthetic provider to develop a personalized treatment in each layer of the skin, delivering robust efficacious clinical results. First-to-market, ultrasound-based imaging and visualization of the epidermis and dermis provide personalized depth measurements to determine the optimal needle depth, eliminating a large portion of guesswork. Previous to this innovation, treatment parameters were fixed, and providers had no visual insight into the dermis or epidermis. A recent clinical study conducted by research dermatologist, Zoe Diana Draelos, MD, was performed to determine the calibration accuracy and the ability for the Vivace Ultra™ to visualize all layers of skin. Thirty (30) healthy individuals, male and female, eighteen (18) years or older were empaneled. Five (5) subjects of each Fitzpatrick Skin Type I-VI were included in the clinical study. The ultrasound scan examined thirty-one (31) anatomical parts of the body. Measurements were taken at baseline and at six (6) weeks. Three (3) measurements were taken at each site. The overall results demonstrated highly reproducible and reliable images across a diverse human subject base.
Upon FDA clearance, Vivace Ultra™ will offer the largest variety of frequency options of any RF microneedling device and insulated and non-insulated cartridges for smaller precise areas as well as larger areas, with the ability to reach a 4.0mm (0.1mm step) depth range. An exclusive new feature available solely with Vivace Ultra® is a patent pending uniform radiofrequency microneedling delivery system designed to evenly distribute heat energy. This feature coupled with precise delivery of energy into the dermis provides a large leap forward in treatment accuracy. Further, medical aesthetic providers will have the ability to store treatment data on a variety of areas and receive recommendations for optimal treatment. Patient experience and colorblind versatility is core to Aesthetics Biomedical’s legacy Vivace Experience® branding and treatment, so naturally Vivace Ultra™ is virtually pain-free for the patient, and effective for light to dark skin types. In addition to visualization, the ultrasound software offers HIPAA compliant cloud connectivity harnessing the ability to collect data about the patient’s various treatment areas beyond the surface for an optimized treatment plan.
Aesthetics Biomedical® is pleased to announce that Vivace Ultra™, based on the receipt of FDA Clearance, is expected to roll-out via limited commercial release in the fall confirming and highlighting anticipation by plastic surgeons, dermatologists, and medical aesthetic practitioners across the country. Vivace Ultra™ will build upon the legacy Vivace® Microneedle RF brand and treatment and provide new opportunities for aesthetic practitioners to advance clinical outcomes and elevate consumer experiences with unmatched precision, visualization, and the most versatile RF microneedling technology available. Vivace Ultra™ is awaiting clearance by the Food & Drug Administration (FDA). Not currently available for sale in the United States of America. For more updates, please visit AestheticsBiomedical.com.
Vivace Ultra® from https://www.aestheticsbiomedical.com/ is awaiting clearance by the Food & Drug Administration (FDA). Not available for sale in the United States of America.
Vivace Ultra™, now awaiting FDA Clearance, provides physicians and aesthetic practitioners unmatched precision, visualization, and the most versatile radiofrequency (RF) microneedling technology available. An innovative reimagination and technical upgrade of the legacy Vivace® Microneedle RF treatment and device, the Vivace Ultra™ combines two unique modalities into one compact device, delivering the most bespoke, personalized treatment experience possible. This new era of advanced energy-based technology offers data-supported treatment parameters made possible by an industry-first ultrasound imaging and mapping, an exclusive uniform radiofrequency microneedling energy delivery system, range of frequency options, and a HIPAA compliant patient data-tracking system for optimal consumer results and treatment plans. Without a doubt, Vivace Ultra™ is the next generation of the Vivace® Microneedle RF.
A recent clinical study found that 95% of patients noticed an improvement in skin texture after a series of treatments between 2-6 sessions. 94% of these patients said they would recommend radiofrequency microneedling to a friend1. On the RealSelf platform, the legacy Vivace® Microneedle RF treatment has become the most requested microneedling treatment2. Building upon an already best-in-class device, Vivace Ultra™ has been developed alongside and in collaboration with key opinion leaders. The new device’s technology and user-friendliness is based on years of clinical and user feedback from top plastic surgeons, dermatologists and medical aesthetic practitioners.
Sleek and simplistically styled, the Vivace Ultra™ is developed and manufactured by Aesthetics Biomedical® Inc., leading the market as the first and only RF Microneedling device manufactured in the United States of America, Phoenix, Arizona. Vivace Ultra™ uses linear array ultrasound technology to visually map the skin across its large 21.5" display screen, allowing an aesthetic provider to develop a personalized treatment in each layer of the skin, delivering robust efficacious clinical results. First-to-market, ultrasound-based imaging and visualization of the epidermis and dermis provide personalized depth measurements to determine the optimal needle depth, eliminating a large portion of guesswork. Previous to this innovation, treatment parameters were fixed, and providers had no visual insight into the dermis or epidermis. A recent clinical study conducted by research dermatologist, Zoe Diana Draelos, MD, was performed to determine the calibration accuracy and the ability for the Vivace Ultra™ to visualize all layers of skin. Thirty (30) healthy individuals, male and female, eighteen (18) years or older were empaneled. Five (5) subjects of each Fitzpatrick Skin Type I-VI were included in the clinical study. The ultrasound scan examined thirty-one (31) anatomical parts of the body. Measurements were taken at baseline and at six (6) weeks. Three (3) measurements were taken at each site. The overall results demonstrated highly reproducible and reliable images across a diverse human subject base.
Upon FDA clearance, Vivace Ultra™ will offer the largest variety of frequency options of any RF microneedling device and insulated and non-insulated cartridges for smaller precise areas as well as larger areas, with the ability to reach a 4.0mm (0.1mm step) depth range. An exclusive new feature available solely with Vivace Ultra® is a patent pending uniform radiofrequency microneedling delivery system designed to evenly distribute heat energy. This feature coupled with precise delivery of energy into the dermis provides a large leap forward in treatment accuracy. Further, medical aesthetic providers will have the ability to store treatment data on a variety of areas and receive recommendations for optimal treatment. Patient experience and colorblind versatility is core to Aesthetics Biomedical’s legacy Vivace Experience® branding and treatment, so naturally Vivace Ultra™ is virtually pain-free for the patient, and effective for light to dark skin types. In addition to visualization, the ultrasound software offers HIPAA compliant cloud connectivity harnessing the ability to collect data about the patient’s various treatment areas beyond the surface for an optimized treatment plan.
Aesthetics Biomedical® is pleased to announce that Vivace Ultra™, based on the receipt of FDA Clearance, is expected to roll-out via limited commercial release in the fall confirming and highlighting anticipation by plastic surgeons, dermatologists, and medical aesthetic practitioners across the country. Vivace Ultra™ will build upon the legacy Vivace® Microneedle RF brand and treatment and provide new opportunities for aesthetic practitioners to advance clinical outcomes and elevate consumer experiences with unmatched precision, visualization, and the most versatile RF microneedling technology available. Vivace Ultra™ is awaiting clearance by the Food & Drug Administration (FDA). Not currently available for sale in the United States of America. For more updates, please visit AestheticsBiomedical.com.
Vivace Ultra® from https://www.aestheticsbiomedical.com/ is awaiting clearance by the Food & Drug Administration (FDA). Not available for sale in the United States of America.
Contact
bluPRint
Jill Eisenstadt Chayet
310-650-2900
www.blupr.com
Jill Eisenstadt Chayet
310-650-2900
www.blupr.com
Categories
