SIMSTECH AI Tracking Technology Captures Major Market Attention; Targets Q4 EU Partnership
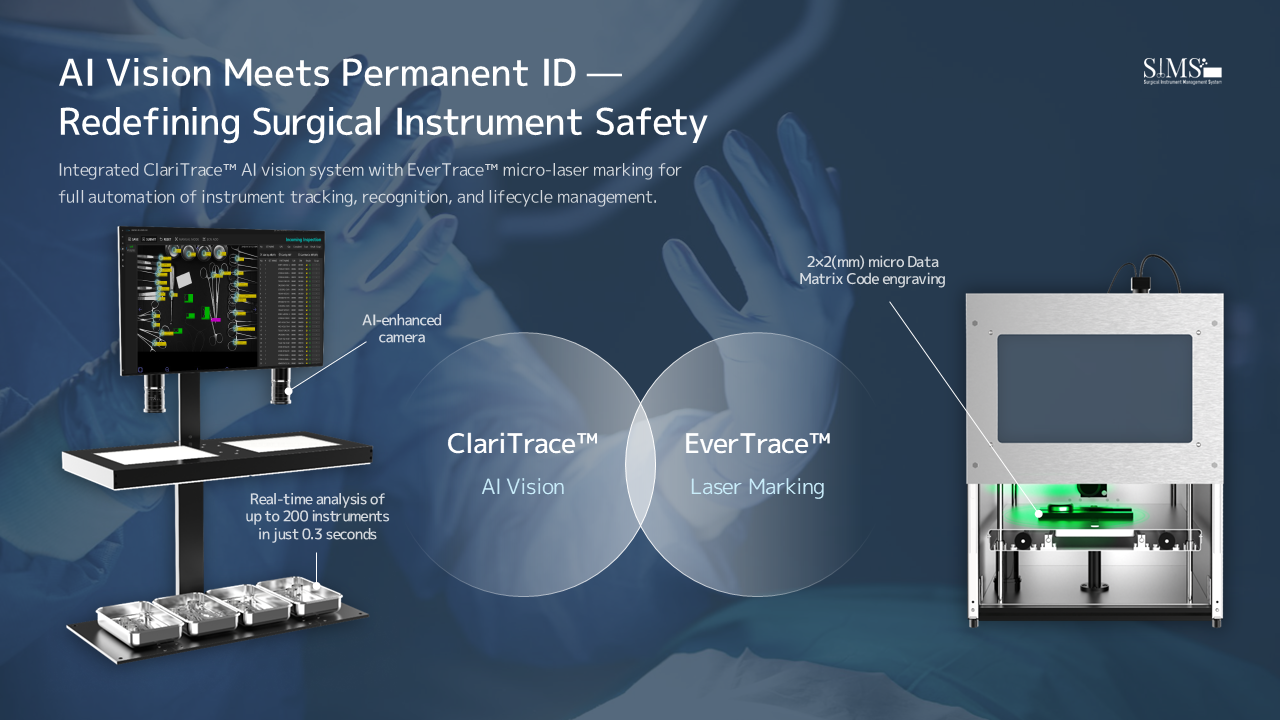
Los Angeles, CA, September 28, 2025 --(PR.com)-- SIMSTECH, a leading developer of AI-driven reusable surgical instrument tracking solutions, showcased its ClariTrace™ and EverTrace™ systems, which are defining a new benchmark for surgical instrument traceability. At two simultaneous major exhibitions—the Gangwon Medical Equipment Show (GMES 2025) and the K-Hospital+Healthtech Fair (KHF 2025) (September 18–19)—the company secured substantial interest from both domestic and international buyers, reflecting a critical global shift toward comprehensive hospital asset management and patient safety mandates.
Global Demand Confirmed at GMES 2025
The GMES 2025 exhibition, co-hosted by WMIT and KOTRA, was the largest in its history, featuring 139 exhibiting companies and drawing 1,790 visitors, including 149 overseas buyers. At the SIMSTECH booth, the company demonstrated ClariTrace™ and EverTrace™, its AI-based surgical instrument identification systems. European buyers expressed considerable interest in the technology's rapid and accurate instrument recognition and its full-process tracking management, citing these capabilities as essential for meeting the stringent EU MDR traceability requirements.
Conversely, the gradually increasing need for comprehensive hospital asset management in Southeast Asian markets, particularly the Philippines and Vietnam, prompted a series of focused meetings.
Growing Policy Discussions in Korea
During the same period, at KHF 2025 in Seoul, organized by the Korean Hospital Association, SIMSTECH drew a favorable response from a wide range of medical professionals, including staff from Korea's prestigious large university hospitals and mid-sized hospitals. While surgical instrument tracking is not yet a legal mandate in Korea, the event highlighted the increasing importance of infection control and hospital asset management. This focus coincides with growing legislative demands for patient safety, which are driving active discussions within the National Assembly and relevant administrative bodies in Korea regarding the reuse of surgical instruments and operating room management.
SIMSTECH’s Strategic Positioning & Partnership Outlook
Across the two shows, SIMSTECH secured over 30 meetings with potential customers, demonstrating strong market demand and the competitiveness of its solutions. Shinhwan Cho, CEO of SIMSTECH, noted that their technologies, ClariTrace™ and EverTrace™, have consistently affirmed technological appeal at more than 20 domestic and international exhibitions since 2024. He added, “Our systems are already fully compliant with EU MDR and U.S. FDA UDI, positioning us to meet—and drive—the critical demands for both Korean and international legislative compliance in surgical asset management.”
For its strategic outlook, SIMSTECH plans to aggressively pursue international market expansion. Cho announced the company will continue its global promotion efforts with participation at major exhibitions, including MEDICA 2025 in Germany this November and CES 2026 in Las Vegas. Furthermore, the company is aiming to finalize a strategic partnership with a major European medical corporation by the fourth quarter of 2025, which is key to securing its long-term global position.
About SIMSTECH
SIMSTECH is a Korea-based medical IoT company specializing in AI-powered tracking and lifecycle management solutions for reusable surgical instruments. Its flagship technologies, ClariTrace™ and EverTrace™, enable rapid, high-accuracy recognition and permanent marking of surgical instruments, enhancing patient safety, operational efficiency, and compliance with global regulatory standards.
Global Demand Confirmed at GMES 2025
The GMES 2025 exhibition, co-hosted by WMIT and KOTRA, was the largest in its history, featuring 139 exhibiting companies and drawing 1,790 visitors, including 149 overseas buyers. At the SIMSTECH booth, the company demonstrated ClariTrace™ and EverTrace™, its AI-based surgical instrument identification systems. European buyers expressed considerable interest in the technology's rapid and accurate instrument recognition and its full-process tracking management, citing these capabilities as essential for meeting the stringent EU MDR traceability requirements.
Conversely, the gradually increasing need for comprehensive hospital asset management in Southeast Asian markets, particularly the Philippines and Vietnam, prompted a series of focused meetings.
Growing Policy Discussions in Korea
During the same period, at KHF 2025 in Seoul, organized by the Korean Hospital Association, SIMSTECH drew a favorable response from a wide range of medical professionals, including staff from Korea's prestigious large university hospitals and mid-sized hospitals. While surgical instrument tracking is not yet a legal mandate in Korea, the event highlighted the increasing importance of infection control and hospital asset management. This focus coincides with growing legislative demands for patient safety, which are driving active discussions within the National Assembly and relevant administrative bodies in Korea regarding the reuse of surgical instruments and operating room management.
SIMSTECH’s Strategic Positioning & Partnership Outlook
Across the two shows, SIMSTECH secured over 30 meetings with potential customers, demonstrating strong market demand and the competitiveness of its solutions. Shinhwan Cho, CEO of SIMSTECH, noted that their technologies, ClariTrace™ and EverTrace™, have consistently affirmed technological appeal at more than 20 domestic and international exhibitions since 2024. He added, “Our systems are already fully compliant with EU MDR and U.S. FDA UDI, positioning us to meet—and drive—the critical demands for both Korean and international legislative compliance in surgical asset management.”
For its strategic outlook, SIMSTECH plans to aggressively pursue international market expansion. Cho announced the company will continue its global promotion efforts with participation at major exhibitions, including MEDICA 2025 in Germany this November and CES 2026 in Las Vegas. Furthermore, the company is aiming to finalize a strategic partnership with a major European medical corporation by the fourth quarter of 2025, which is key to securing its long-term global position.
About SIMSTECH
SIMSTECH is a Korea-based medical IoT company specializing in AI-powered tracking and lifecycle management solutions for reusable surgical instruments. Its flagship technologies, ClariTrace™ and EverTrace™, enable rapid, high-accuracy recognition and permanent marking of surgical instruments, enhancing patient safety, operational efficiency, and compliance with global regulatory standards.
Contact
SIMSTECH Inc.
Insoo Jeon
+821054484763
www.simstech.co.kr
Insoo Jeon
+821054484763
www.simstech.co.kr
Categories
