Aatru Medical Announces FDA Clearance and Commercial Launch of the NPSIMS™ - Negative Pressure Surgical Incision Management System
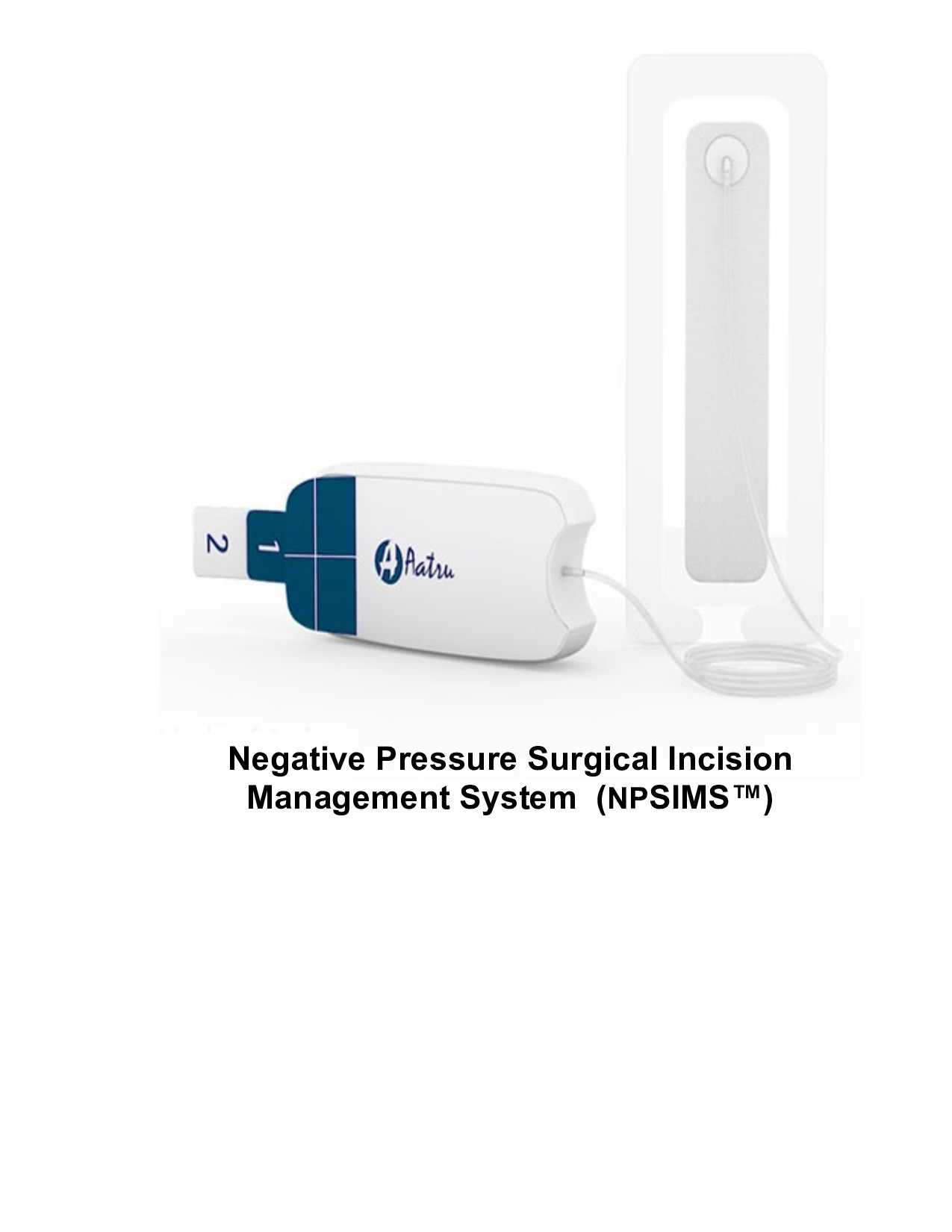
Aatru Medical, LLC announced U.S. Food and Drug Administration (FDA) 510(k) Class II clearance of the NPSIMS™ Negative Pressure Surgical Incision Management System. The NPSIMS utilizes an innovative mode-of-action that eliminates the expensive electro-mechanical pump, battery, and electronics found in most other negative pressure wound therapy (NPWT) systems being deployed for closed surgical incision applications.
Cleveland, OH, August 24, 2021 --(PR.com)-- Aatru Medical, LLC ("Aatru") today announced U.S. Food and Drug Administration (FDA) 510(k) Class II clearance of the NPSIMS™ Negative Pressure Surgical Incision Management System.
The NPSIMS utilizes an innovative mode-of-action that eliminates the expensive electro-mechanical pump, battery, and electronics found in most other negative pressure wound therapy (NPWT) systems being deployed for closed surgical incision applications. The single-use, disposable NPSIMS is easily activated and operates silently during therapy delivery. A patented preassembled dressing provides ease-of-application with exceptional "sealing" capability while providing patient comfort and ergonometric appeal.
The FDA effort was led by Aatru President and Board member Edward Armstrong who leveraged his extensive professional prior experience with NPWT and medical device companies. “This innovative NPWT technology allows the NPSIMS product to provide clinical, patient and economic benefits in an elegant presentation, which we look forward to making available to users in the near future,” said Mr. Armstrong.
“In spite of the significant challenges posed by the global pandemic, the Aatru team with the support of our regulatory partners successfully received FDA Class II clearance for the NPSIMS. We believe that we are now well-positioned to commercially launch in multiple countries that have expressed immediate interest in our NPWT platform. We have filed more than 60 patents globally to protect our innovative technology, and we have identified manufacturing partners who can immediately scale to meet global demand,” said Aatru’s CEO and Chairman, Timothy Wojciechowski.
About NPWT for closed surgical incisions:
Several post-operative wound complications are common following surgical procedures. Negative pressure wound therapy (NPWT) is well recognized for the management of open wounds, and in the last several years has been applied to closed surgical incisions. Compared with standard postoperative dressings, NPWT significantly reduces the rate of wound infection and seroma. The global market opportunity for NPWT in the closed surgical incision market is approximately $12B annually with low single-digit market penetration to date.
About Aatru Medical:
Aatru is a privately held medical device technology company focused on disrupting the surgical incision market with its NPSIMS, a simple, disposable, single-use, patented, low-cost NPWT device, uniquely designed to require no electromechanical pump, battery or canister.
An introductory video of the NPSIMS product can be found on the Aatru Medical, LLC website: www.aatru.com.
Contact: Tom Lash
Aatru Medical, LLC Chief Operating Officer
216-303-6063
tlash@aatru.com
The NPSIMS utilizes an innovative mode-of-action that eliminates the expensive electro-mechanical pump, battery, and electronics found in most other negative pressure wound therapy (NPWT) systems being deployed for closed surgical incision applications. The single-use, disposable NPSIMS is easily activated and operates silently during therapy delivery. A patented preassembled dressing provides ease-of-application with exceptional "sealing" capability while providing patient comfort and ergonometric appeal.
The FDA effort was led by Aatru President and Board member Edward Armstrong who leveraged his extensive professional prior experience with NPWT and medical device companies. “This innovative NPWT technology allows the NPSIMS product to provide clinical, patient and economic benefits in an elegant presentation, which we look forward to making available to users in the near future,” said Mr. Armstrong.
“In spite of the significant challenges posed by the global pandemic, the Aatru team with the support of our regulatory partners successfully received FDA Class II clearance for the NPSIMS. We believe that we are now well-positioned to commercially launch in multiple countries that have expressed immediate interest in our NPWT platform. We have filed more than 60 patents globally to protect our innovative technology, and we have identified manufacturing partners who can immediately scale to meet global demand,” said Aatru’s CEO and Chairman, Timothy Wojciechowski.
About NPWT for closed surgical incisions:
Several post-operative wound complications are common following surgical procedures. Negative pressure wound therapy (NPWT) is well recognized for the management of open wounds, and in the last several years has been applied to closed surgical incisions. Compared with standard postoperative dressings, NPWT significantly reduces the rate of wound infection and seroma. The global market opportunity for NPWT in the closed surgical incision market is approximately $12B annually with low single-digit market penetration to date.
About Aatru Medical:
Aatru is a privately held medical device technology company focused on disrupting the surgical incision market with its NPSIMS, a simple, disposable, single-use, patented, low-cost NPWT device, uniquely designed to require no electromechanical pump, battery or canister.
An introductory video of the NPSIMS product can be found on the Aatru Medical, LLC website: www.aatru.com.
Contact: Tom Lash
Aatru Medical, LLC Chief Operating Officer
216-303-6063
tlash@aatru.com
Contact
Aatru Medical, LLC
Tom Lash
216-303-6063
www.aatru.com
Tom Lash
216-303-6063
www.aatru.com
Categories
