US Patent Granted for the WELLEX™ Interspinous Technology
A Key US Patent for the Treatment of Spinal Stenosis
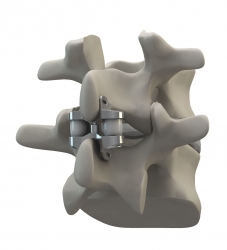
Altamonte Springs, FL, September 26, 2011 --(PR.com)-- Eden Spine (www.EdenSpine.com), a Florida based Medical Devices organization focused on developing cutting edge spinal technologies, announced today that on September 20th, 2011, it was granted US Patent No 8,021,395 from the U.S. Patent & Trademark Office (USPTO) for its Dynamic Compressible Extension Controller, the WELLEX™.
“The WELLEX™, developed in partnership with Dr. Fuentes, Neurosurgeon, is a non-fusion technology that addresses the needs of the ever growing population of aging patients around the world suffering from Spinal Stenosis, but not willing to suffer the consequences of a spinal fusion,” says Mourad Ben Mokhtar, Head of Eden Spine’s R&D efforts. He adds, “We already have the technologies to remove pain by fusing the spine. The true challenge is to remove pain while protecting the spine, and maintaining the functionality of the diseased motion segment, for better long-term results. I am proud to say, that this is what we have accomplished with the WELLEX.”
The WELLEX™ Interspinous technology fills the gap between less effective conservative care and riskier fusion. It is typically a 30 minute low risk procedure that requires an incision of less than one inch. Predominantly, patients are capable of going home on the day of the surgery and are back to normal activities within 4 weeks. The uniqueness of the technology comes from its ability to remove pain and respect the kinematics of the motion segment.
According to Guillaume Viallaneix, CEO, “The WELLEX™ has immense market potential for 3 reasons (i) Spinal Stenosis is the #1 pre-op diagnosis for adults over 65 (ii) an aging population is the #1 reason for the increase of spinal stenosis (iii) in the US alone, by 2026, 20% of the population, or 65 million people will be over the age of 65, with an estimated 8% suffering from spinal stenosis. There is an unmet clinical need that the WELLEX™ addresses, as patients in the US and around the world are and will be longing for minimally invasive solutions that can remove their pain without fusing their spine.”
Invented in 2006, tested in 2007, the WELLEX™ obtained CE Mark in 2008 and was launched clinically in 2009. Eden Spine is currently gathering clinical data helping patients daily in 15 markets around the world. “This new Patent is a milestone in building our IP portfolio,” says Guillaume Viallaneix, CEO, “ideally positioning Eden Spine for long term growth and value creation.”
###
About Eden Spine: Eden Spine LLC is a privately held, technology driven, spine organization. The Eden Spine Group is headquartered in Florida, with a wholly owned subsidiary in Geneva, Switzerland. It is currently developing and distributing a range of new generation dynamic spinal technologies. Eden Spine is present in the United States, Europe, the Middle East and Latin America. The company possesses 5 proprietary technologies including three new-generation, motion preservation technologies; the WELLDISC™ total disc replacement; the PERFX-2™ Dynamic Stabilization System, and the WELLEX™ Interspinous Technology. Eden Spine currently distributes a range of FDA-cleared and CE Marked spine technologies in the US and internationally.
“The WELLEX™, developed in partnership with Dr. Fuentes, Neurosurgeon, is a non-fusion technology that addresses the needs of the ever growing population of aging patients around the world suffering from Spinal Stenosis, but not willing to suffer the consequences of a spinal fusion,” says Mourad Ben Mokhtar, Head of Eden Spine’s R&D efforts. He adds, “We already have the technologies to remove pain by fusing the spine. The true challenge is to remove pain while protecting the spine, and maintaining the functionality of the diseased motion segment, for better long-term results. I am proud to say, that this is what we have accomplished with the WELLEX.”
The WELLEX™ Interspinous technology fills the gap between less effective conservative care and riskier fusion. It is typically a 30 minute low risk procedure that requires an incision of less than one inch. Predominantly, patients are capable of going home on the day of the surgery and are back to normal activities within 4 weeks. The uniqueness of the technology comes from its ability to remove pain and respect the kinematics of the motion segment.
According to Guillaume Viallaneix, CEO, “The WELLEX™ has immense market potential for 3 reasons (i) Spinal Stenosis is the #1 pre-op diagnosis for adults over 65 (ii) an aging population is the #1 reason for the increase of spinal stenosis (iii) in the US alone, by 2026, 20% of the population, or 65 million people will be over the age of 65, with an estimated 8% suffering from spinal stenosis. There is an unmet clinical need that the WELLEX™ addresses, as patients in the US and around the world are and will be longing for minimally invasive solutions that can remove their pain without fusing their spine.”
Invented in 2006, tested in 2007, the WELLEX™ obtained CE Mark in 2008 and was launched clinically in 2009. Eden Spine is currently gathering clinical data helping patients daily in 15 markets around the world. “This new Patent is a milestone in building our IP portfolio,” says Guillaume Viallaneix, CEO, “ideally positioning Eden Spine for long term growth and value creation.”
###
About Eden Spine: Eden Spine LLC is a privately held, technology driven, spine organization. The Eden Spine Group is headquartered in Florida, with a wholly owned subsidiary in Geneva, Switzerland. It is currently developing and distributing a range of new generation dynamic spinal technologies. Eden Spine is present in the United States, Europe, the Middle East and Latin America. The company possesses 5 proprietary technologies including three new-generation, motion preservation technologies; the WELLDISC™ total disc replacement; the PERFX-2™ Dynamic Stabilization System, and the WELLEX™ Interspinous Technology. Eden Spine currently distributes a range of FDA-cleared and CE Marked spine technologies in the US and internationally.
Contact
Eden Spine, LLC
Guillaume Viallaneix
407-900-9986
www.EdenSpine.com
Guillaume Viallaneix
407-900-9986
www.EdenSpine.com
Multimedia

North American Spine Society
For more information about the WELLEX, visit Eden Spine at NASS booth 2622.
Categories

