New Rapid COVID-19 Point of Care Test
Pepex Biomedical has diverted funds and resources to the development of Multiple Rapid COVID-19 Tests designed to accurately identify Antigens (diagnostic to detect active virus) and Antibodies (to determine immunity) in under 15 minutes with 95% accuracy.
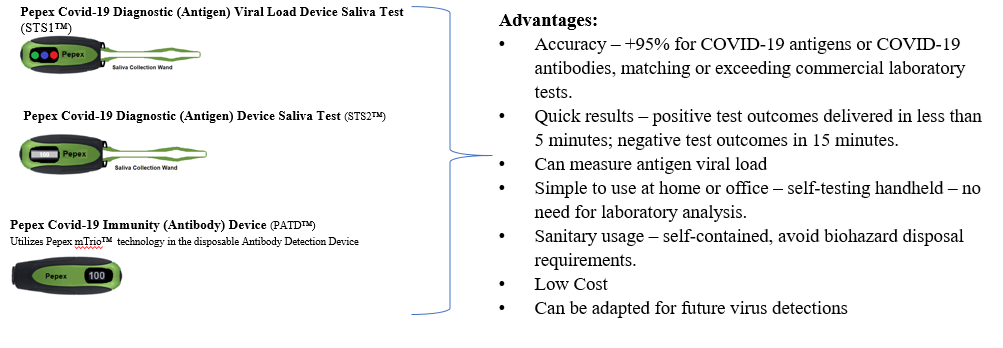
Atlanta, GA, November 09, 2020 --(PR.com)-- Pepex Biomedical, Inc. is a leading developer of enzymatic biosensor technology focused on enabling healthier lives across the globe. Historically focused on diagnostic blood glucose measuring systems, Pepex has recently pivoted to apply its proprietary sensor platform to the development of immunological sensors specific to COVID-19 virus and the worldwide pandemic. The Company is preparing to commercialize four COVID-19 test products designed to provide safe, accurate Antigen and Antibody measurement results.
The scope of the project includes four self-administered devices that include two saliva based sensors - one that will detect the presence of the COVID-19 virus in less than 15 minutes at a greater than 95% accuracy, and a second sensor device with the ability to quantify the viral load. The other two devices use Pepex novel sensor technology to detect antibodies in interstitial fluid for presence, and the other to quantify titers, rapidly and accurately.
All four test devices are self-contained and do not require additional diagnostic equipment or laboratory assistance.
Statement from Paul Danner, Chief Executive Officer - “We believe our suite of COVID-19 test products will provide a significant positive impact on the global effort to bring the pandemic under control. There are currently no test systems like these in the marketplace that are simple to self-administer, safely disposable, inexpensive, and reliable. By utilizing Pepex’s patented CCM® fiber technology, we expect to be able to match or exceed commercial laboratory test result accuracy.”
Pepex intends to seek Emergency Use Authorization (EUA) authority from the Food and Drug Administration (FDA) during the first quarter 2021.
Pepex has retained the services of Evangelyn Alocilja, PhD, a Professor at Michigan State University, as lead Immunologist. Located adjacent to Pepex’s lab in East Lansing, Michigan, Dr. Alocilja is an expert in infectious diseases with 8 patents and 11 patent applications, several related to gold nanoparticles at the core of Pepex’s three-electrode sensor technology. In addition, Vojtech Svoboda, PhD, Pepex’s lead scientist, provides distinguished expertise in electrochemistry. Dr. Svoboda is credited as the key contributor to the development of Pepex’s three-electrode sensor technology.
Manufacturing Partners:
Pepex has partnered with Insight Innovation Center-Chicago on previous projects, and has once again retained them to assist in the industrial design of the COVID-19 devices including collaboration on design development, production tooling and design for manufacture (DFM) elements. Insight Innovation Center will provide Pepex with end-to-end development services from early to late-stage with design research, user experience, engineering, and human factors requirements. Insight’s parent company is Nemera.
Upon completion of DFM, Pepex intends to use Nemera as its premier contract manufacturer to produce the COVID-19 devices. Nemera is a world leader in the design, development, and manufacturing of drug delivery devices for the pharmaceutical, biotechnology and generics industries.
In addition, Pepex has selected Mikron Manufacturing to design and build the high-volume automated manufacturing equipment required to produce the COVID-19 devices. Pepex is also working with Mikron on its blood glucose device. The Mikron Group develops, produces and markets highly precise, productive and adaptable automation solutions.
About Pepex Biomedical, Inc.
Pepex Biomedical (https://www.pepex.com/) is a privately held company seeking additional funding to complete its COVID-19 project. The Company has an extensive portfolio of over 300 global patents that protect proprietary manufacturing processes and unique biosensor designs for a broad base of applications including COVID-19, Sepsis, HIV, Ebola, Hepatitis, Lactate, Blood Sugar and other life-altering biomarkers. Pepex Biomedical’s mission is to become the leading technology source, prime supplier, and marketer of enzymatic and immunological biosensors for consumer medical devices and hospital diagnostic use.
For more information, please contact Paul Danner, Chief Executive Officer, at paul.danner@pepex.com or +1.401.588.0110.
The scope of the project includes four self-administered devices that include two saliva based sensors - one that will detect the presence of the COVID-19 virus in less than 15 minutes at a greater than 95% accuracy, and a second sensor device with the ability to quantify the viral load. The other two devices use Pepex novel sensor technology to detect antibodies in interstitial fluid for presence, and the other to quantify titers, rapidly and accurately.
All four test devices are self-contained and do not require additional diagnostic equipment or laboratory assistance.
Statement from Paul Danner, Chief Executive Officer - “We believe our suite of COVID-19 test products will provide a significant positive impact on the global effort to bring the pandemic under control. There are currently no test systems like these in the marketplace that are simple to self-administer, safely disposable, inexpensive, and reliable. By utilizing Pepex’s patented CCM® fiber technology, we expect to be able to match or exceed commercial laboratory test result accuracy.”
Pepex intends to seek Emergency Use Authorization (EUA) authority from the Food and Drug Administration (FDA) during the first quarter 2021.
Pepex has retained the services of Evangelyn Alocilja, PhD, a Professor at Michigan State University, as lead Immunologist. Located adjacent to Pepex’s lab in East Lansing, Michigan, Dr. Alocilja is an expert in infectious diseases with 8 patents and 11 patent applications, several related to gold nanoparticles at the core of Pepex’s three-electrode sensor technology. In addition, Vojtech Svoboda, PhD, Pepex’s lead scientist, provides distinguished expertise in electrochemistry. Dr. Svoboda is credited as the key contributor to the development of Pepex’s three-electrode sensor technology.
Manufacturing Partners:
Pepex has partnered with Insight Innovation Center-Chicago on previous projects, and has once again retained them to assist in the industrial design of the COVID-19 devices including collaboration on design development, production tooling and design for manufacture (DFM) elements. Insight Innovation Center will provide Pepex with end-to-end development services from early to late-stage with design research, user experience, engineering, and human factors requirements. Insight’s parent company is Nemera.
Upon completion of DFM, Pepex intends to use Nemera as its premier contract manufacturer to produce the COVID-19 devices. Nemera is a world leader in the design, development, and manufacturing of drug delivery devices for the pharmaceutical, biotechnology and generics industries.
In addition, Pepex has selected Mikron Manufacturing to design and build the high-volume automated manufacturing equipment required to produce the COVID-19 devices. Pepex is also working with Mikron on its blood glucose device. The Mikron Group develops, produces and markets highly precise, productive and adaptable automation solutions.
About Pepex Biomedical, Inc.
Pepex Biomedical (https://www.pepex.com/) is a privately held company seeking additional funding to complete its COVID-19 project. The Company has an extensive portfolio of over 300 global patents that protect proprietary manufacturing processes and unique biosensor designs for a broad base of applications including COVID-19, Sepsis, HIV, Ebola, Hepatitis, Lactate, Blood Sugar and other life-altering biomarkers. Pepex Biomedical’s mission is to become the leading technology source, prime supplier, and marketer of enzymatic and immunological biosensors for consumer medical devices and hospital diagnostic use.
For more information, please contact Paul Danner, Chief Executive Officer, at paul.danner@pepex.com or +1.401.588.0110.
Contact
Pepex Biomedical, Inc.
Paul K. Danner
401.588.0110
https://www.pepex.com
Paul K. Danner
401.588.0110
https://www.pepex.com
Categories
