Phase One Development of Rapid COVID-19 Point of Care Test Complete
Pepex Biomedical has effectively concluded first stage development efforts including successfully demonstrating working chemistry and manufacturing functionality of its existing electrochemical biosensor.
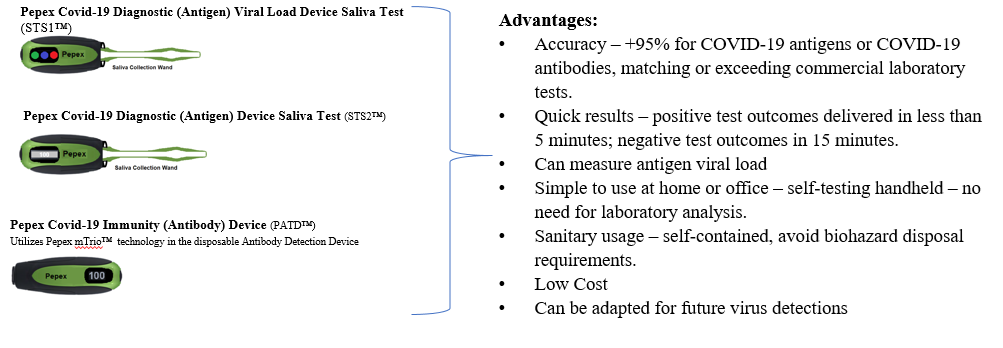
Atlanta, GA, February 11, 2021 --(PR.com)-- Pepex Biomedical, Inc. (the “Company”), a leading developer of enzymatic biosensor technology, has completed the initial development phase of its forthcoming suite of immunosensor testing products designed to accurately identify COVID-19 antigens and antibodies in under 15 minutes with 95% accuracy.
The scope of the project includes at least four self-administered devices that include two saliva-based sensors; one that will detect the presence of the COVID-19 virus in less than 15 minutes at a greater than 95% accuracy, and a second sensor device with the ability to quantify the viral load. The other two devices use Pepex novel sensor technology to detect antibodies in interstitial fluid for presence, and the other to quantify titers, rapidly and accurately.
The Company has made significant progress the past 90 days, and is now nearing the point of commercializing four COVID-19 test products designed to provide safe, accurate antigen and antibody measurement results. Each of the devices is entirely self-contained, and do not require any additional diagnostic equipment or laboratory assistance to produce rapid, reliable results.
“I am very pleased to report that over the past few months our technical team successfully demonstrated our ability to detect the presence of COVID-19 antigens and antibodies, and that the envisioned test products can indeed be mass produced by coupling the newly-developed chemistry with our proprietary Aerosol Jet Printing (AJP) biosensor manufacturing techniques,” said Paul Danner, Chief Executive Officer of Pepex Biomedical. “Each of these tests will provide a highly cost-effective, completely self-administered alternative to all of the other systems currently available,” he said.
Based on the successful proof of concept of the sensor designs, the Company will now engage the Food and Drug Administration (FDA) to seek further guidance on obtaining Emergency Use Authorization (EUA), and based historic precedence, believes its COVID-19 test products to be excellent candidates for approval in an effort to battle the current pandemic more effectively.
In Phase II, Pepex in collaboration with its partners at InSight Product Development, will complete a Design for Manufacturing process to optimize the physical form factor in order to minimize cost and increase ease of manufacturing. The Company also intends to conclude final selection of the vendor that will provide requisite AJP equipment for high-volume sensor production, along with all other requirements for full scale manufacturing.
Equally noteworthy, the Company has been recently informed by its intellectual property counsel that patent applications protecting its proprietary AJP techniques have been approved by China, Germany, Luxembourg, the United Kingdom, and 34 other countries around the world.
About Pepex Biomedical, Inc.
Pepex Biomedical (https://www.pepex.com/) is a privately held company seeking additional funding to complete its COVID-19 project. The Company has an extensive portfolio of over 300 global patents that protect proprietary manufacturing processes and unique biosensor designs for a broad base of applications including COVID-19, Sepsis, HIV, Ebola, Hepatitis, Lactate, Blood Sugar, and other life-altering biomarkers. Pepex Biomedical’s mission is to become the leading technology source, prime supplier, and marketer of enzymatic and immunological biosensors for consumer medical devices and hospital diagnostic use.
The scope of the project includes at least four self-administered devices that include two saliva-based sensors; one that will detect the presence of the COVID-19 virus in less than 15 minutes at a greater than 95% accuracy, and a second sensor device with the ability to quantify the viral load. The other two devices use Pepex novel sensor technology to detect antibodies in interstitial fluid for presence, and the other to quantify titers, rapidly and accurately.
The Company has made significant progress the past 90 days, and is now nearing the point of commercializing four COVID-19 test products designed to provide safe, accurate antigen and antibody measurement results. Each of the devices is entirely self-contained, and do not require any additional diagnostic equipment or laboratory assistance to produce rapid, reliable results.
“I am very pleased to report that over the past few months our technical team successfully demonstrated our ability to detect the presence of COVID-19 antigens and antibodies, and that the envisioned test products can indeed be mass produced by coupling the newly-developed chemistry with our proprietary Aerosol Jet Printing (AJP) biosensor manufacturing techniques,” said Paul Danner, Chief Executive Officer of Pepex Biomedical. “Each of these tests will provide a highly cost-effective, completely self-administered alternative to all of the other systems currently available,” he said.
Based on the successful proof of concept of the sensor designs, the Company will now engage the Food and Drug Administration (FDA) to seek further guidance on obtaining Emergency Use Authorization (EUA), and based historic precedence, believes its COVID-19 test products to be excellent candidates for approval in an effort to battle the current pandemic more effectively.
In Phase II, Pepex in collaboration with its partners at InSight Product Development, will complete a Design for Manufacturing process to optimize the physical form factor in order to minimize cost and increase ease of manufacturing. The Company also intends to conclude final selection of the vendor that will provide requisite AJP equipment for high-volume sensor production, along with all other requirements for full scale manufacturing.
Equally noteworthy, the Company has been recently informed by its intellectual property counsel that patent applications protecting its proprietary AJP techniques have been approved by China, Germany, Luxembourg, the United Kingdom, and 34 other countries around the world.
About Pepex Biomedical, Inc.
Pepex Biomedical (https://www.pepex.com/) is a privately held company seeking additional funding to complete its COVID-19 project. The Company has an extensive portfolio of over 300 global patents that protect proprietary manufacturing processes and unique biosensor designs for a broad base of applications including COVID-19, Sepsis, HIV, Ebola, Hepatitis, Lactate, Blood Sugar, and other life-altering biomarkers. Pepex Biomedical’s mission is to become the leading technology source, prime supplier, and marketer of enzymatic and immunological biosensors for consumer medical devices and hospital diagnostic use.
Contact
Pepex Biomedical, Inc.
Paul K. Danner
401.588.0110
https://www.pepex.com
Paul K. Danner
401.588.0110
https://www.pepex.com
Categories
